ALUMINUM SILICATES (compounded aluminum, oxygen and silicate) exist in various useful forms and chemical compositions. They can be roughly divided into categories like anhydrous or hydrated, naturally-occurring minerals or synthetic constructs, and crystalline and amorphous form factors.
Further sub-classifications are useful. For example, crystalline-structured mineral forms of aluminum silicate, like kyanite and sillimanite, share a common chemical formula (Al2SiO5), yet are distinct in structure—which in turn dictate applicable utility. Some aluminum silicate compounds are nuanced by the presence of other chemical influences—for example, a hydrated magnesium aluminum silicate provides use-value in medicinal applications or in beauty products.
As for functional utility, aluminum silicates are widely used in industry. The pharmaceutical industry uses aluminum silicate compounds as demulcents and adsorbents or medical agents. In raw material form, aluminum silicates are used in papers, plastics, high-performance concretes, ceramics, insulators, and insoluble dyes. Aluminum silicates even function effectively in simple roles—spill absorbents, polishing grits, anti-clumping agents, or release-agent coatings. Even as a mechanical insecticide.
As for availability, Alumina (Al2O3) and Silica (SiO2) are two of the most abundant minerals in the earth’s crust.
Form Matters
THE INDUSTRIAL APPLICATIONS for aluminum silicate compounds are constantly expanding, and one of the keys to industrial fit both present and future is the form structure itself: crystalline or amorphous.
CRYSTALLINE: For example, the compound mix and crystalline structure of kyanite-type aluminum silicates are used for ceramics applications. Sillimanite types are common in glass-making and smelting and metal foundry industries. Hydrated aluminum silicates form a group of clay minerals known as Kaolins, which rely on alternating mineral structure to deliver the useful applications.
AMORPHOUS: Aluminum silicates in the safe, non-crystalline form are widely used as a natural pozzolan in concrete mix designs to improve the performance of Portland cements in terms of density, chemical resistance, and enduring strength.
Another application in which the amorphous, sponge-like structured aluminum silicates contribute in form as well as chemical makeup is the coatings industry. Unlike typical platy extender forms such as mica or talc, amorphous forms lock and bind so deeply into the polymer networks of a coating formulation that improvements are realized in scrub and burnish resistance, corrosion and impact resistance, flexibility, film integrity and durability, even color retention. This low-density, frothy amorphous form also allows for volume replacement, which provides considerable cost savings.
Amorphous Means Benign
AMORPHOUS FORMS OF aluminum silicates (like pumice and perlite) pose no hazard for users beyond that of a nuisance dust. Safety data sheets required by OSHA contain no “hazard statements”, no “precautionary statements”, and no “hazards not otherwise classified”. The information presented below is taken from the OSHA-compliant Safety Data Sheet (SDS) issued for all grades of amorphous aluminum silica (pumice form) produced and sold by Hess Pumice Products.
FIRST AID: Inhalation: Remove to fresh air. Drink water to clear throat; blow nose to evacuate dust. Eye Contact: Flush eyes with water. Skin Contact: N/A. Skin Absorption: N/A. Ingestion: N/A.
HANDLING AND STORAGE: Handle in accordance with good industrial hygiene and safety practice. Keep container tightly closed. Avoid contact with eyes.
INCOMPATIBLE MATERIALS: Hydrofluoric Acid. Material reacts with Hydrofluoric Acid to form toxic silicon tetra fluoride gas.
Amorphous Aluminum Silicate in Pumice Form
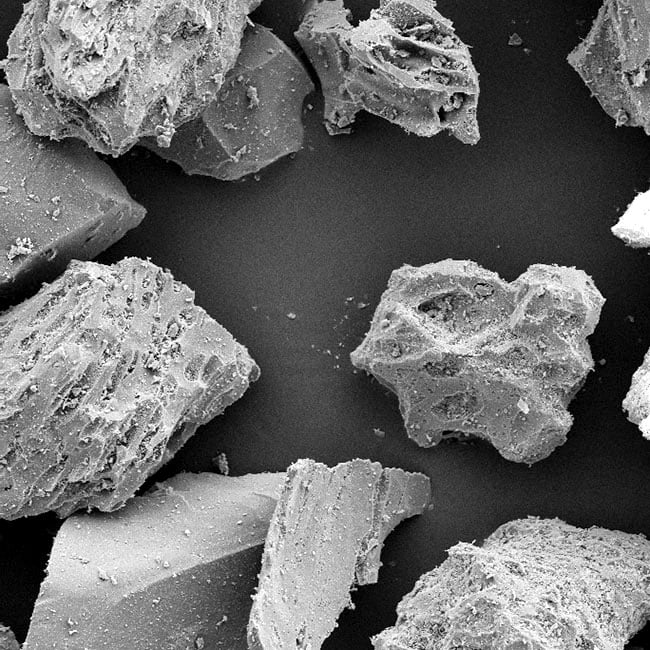
BORN IN THE FIRERY HEART of an ancient volcano, pumice is a mineralized aluminum silicate with an amorphous structure, light and frothy in character (essentially a foamed glass), and so widely useful to industry that it is produced in grades from a lightweight aggregate to an ultrafine powder product of 3 microns.
While pumice is an abundant mineral, it was not all created equal. In the southeast corner of the State of Idaho is an aluminum silicate deposit that lays claim to the world’s purest commercial deposit of white pumice (having been naturally scrubbed of impurities like heavy metals). Hess Pumice Products, of Malad City, Idaho, supplies a wide variety of grades and custom grade blends from this unique deposit to industry worldwide.
Chemical Analysis and Physical Properties
GENERAL DESCRIPTION [ALUMINUM SILICATE, PUMICE TYPE, SE IDAHO DEPOSIT]:
Amorphous (non-crystalline) in structure and composed primarily of aluminum silicate, pumice is a naturally calcined volcanic glass foam consisting of highly vesicular strands permeated with tiny air bubbles. It is these frothy, friable glass vesicles that, when carefully refined to various grades, give pumice its unique and infinitely useful qualities.
CAS NUMBER: 1332-09-8
TYPICAL ANALYSIS:
SILICON DIOXIDE: 73.0-76.0%
ALUMINUM OXIDE: 11.0-14.0%
FERRIC OXIDE: 0.08-1.3%
FERROUS OXIDE: 0.1%
SODIUM OXIDE: 1.0-2.0%
POTASSIUM OXIDE: 1.0-2.0%
CALCIUM OXIDE: 0.5-1.5%
TITANIUM OXIDE: 0-0.4%
MAGNESIUM OXIDE: 0-0.1%
MOISTURE: ‹1.0%
GENERAL PROPERTIES:
CRYSTALLINE SiO2: None Detected.
APPEARANCE: White Powder
HARDNESS (MOHS): 6
pH: 7.2
RADIOACTIVITY: None
SOFTENING POINT: 900 Degrees C
WATER SOLUBLE SUBSTANCES: 0.15%
LOSS ON IGNITION: ‹5.0%
GE BRIGHTNESS: 75-83
SPECIFIC GRAVITY: 2.4
REACTIVITY: Inert
ADDITIONAL INFORMATION and SUPPORT DOCUMENTS:
» Download: Technical Data Sheet (PDF)
» Pumice Safety Data Sheet (PDF)